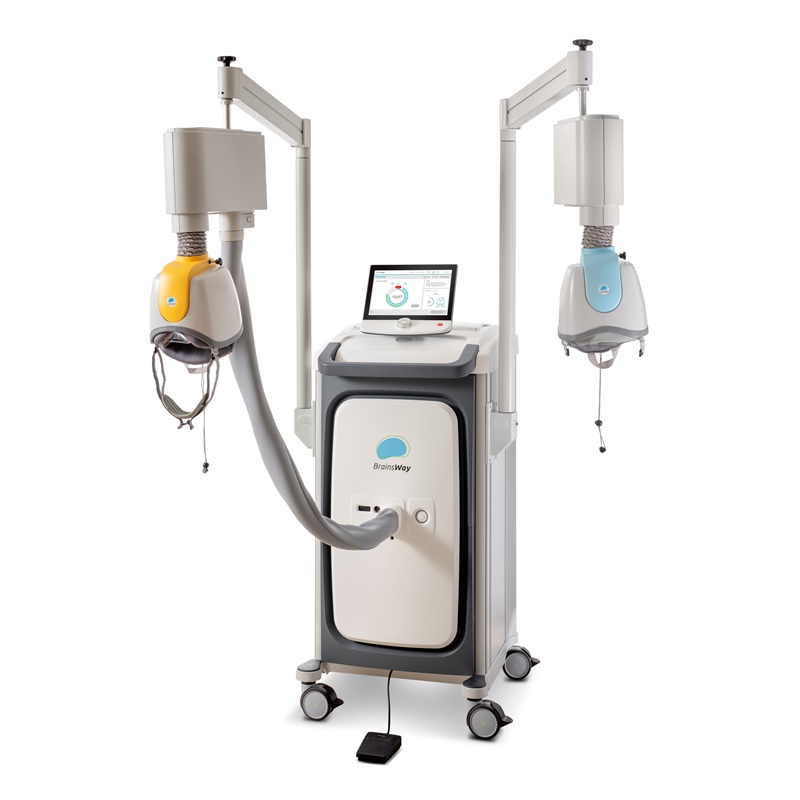
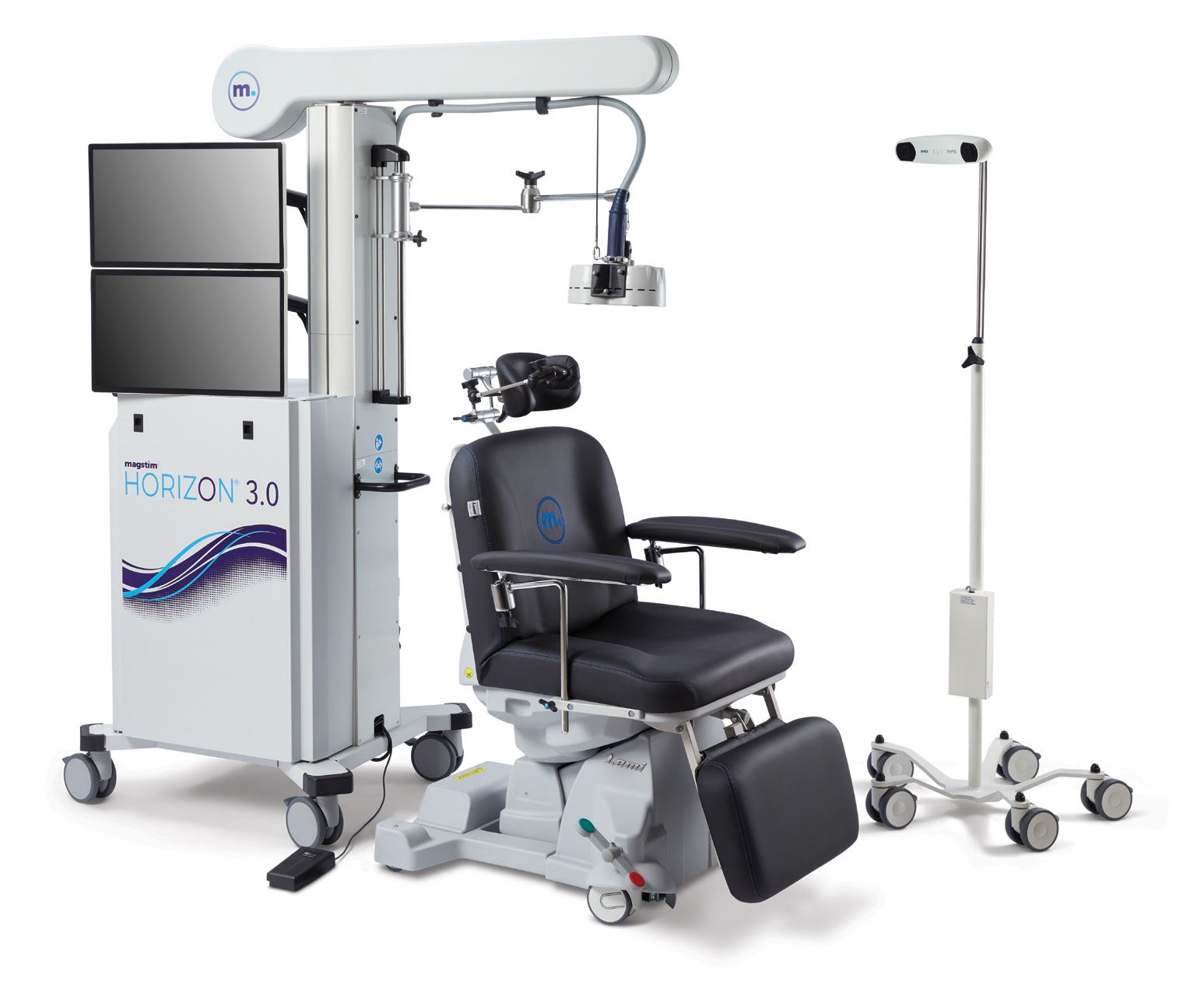
NeuroStar
Neuronetics, Inc., established in 2003, was the first TMS device manufacturer to receive FDA clearance with their NeuroStar system for the treatment of depression in 2008. For some time, Neuronetics was able to dominate the market, as they had no competition. It is important to know that Neuronetics did not invent TMS therapy, as it was initially developed in Europe.
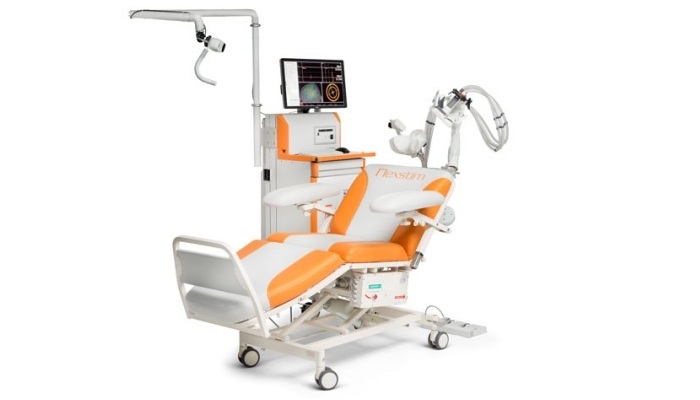
NeuroStar uses a figure-of- 8 coil. The system offers three treatment variations: 1) the standard 37.5-minute session; 2) the DASH 19-minute sessions (it cuts the inter-train intervals from 26 secs to 11 secs); and 3) TouchStar™ Theta Burst, an iTBS protocol. NeuroStar doesn’t have a navigation system to ensure the placement of the coil. Instead, NeuroStar has a "Contact Sensing" to alert the treater if contact is lost.
Neuronetics has a hefty pay-per-use business model, meaning the TMS clinic must pay a fee for every TMS session before able to administer the treatment to the patient. This pay-per-fee use can equate to as much as 25-40% of revenue.
In May 2022, Neuronetics announced an FDA clearance for their F-8 coil to be used for OCD. In July 2022, they received FDA clearance for anxious depression.